【仪器设备:原位X射线衍射仪 配备了转靶和二维Eiger探测器】
【地点:4号楼112室X射线衍射实验室】
理化公共实验平台X射线衍射实验室提供完善的电池材料原位XRD表征支撑,配备大面积的二维面探测器Eiger、变温原位电池的XRD模具、功能强大的数据分析软件以及技术人员丰富的结构精修经验,可极大限度地支持西湖大学相关科研工作。

图1原位XRD配置及电池模具的安装
由于便携式电子设备及电动汽车需求锐增,具有能量密度高、无记忆效应、在不使用时只有缓慢电荷损失的锂离子电池,亟需对其电化学性能进行优化。而影响其电化学性能的最关键因素,便是电极的反应机制。锂离子电池电极材料的性能主要取决于其组成及结构。系统研究电极材料的组成、结构及性能的构效关系,探索电极材料在充放电过程中的结构演化、离子及电荷转移,对深入了解电极材料的储锂机制,及优化材料的化学组成、晶体结构及形貌都具有十分重要的意义。原位X射线衍射技术(in-situ XRD)聚焦于在材料的形成和转化过程中进行实时XRD扫描,如在电池材料充放电过程中,实时监测电极材料相变和结构演变,精确反映电池反应的机制。
由于不同极片间的物理差异性和拆电池、极片洗涤、转移等操作过程的影响,非原位XRD测试往往不能很好地还原电池材料在充放电过程中真实状况,比如不同极片上活性材料的质量和分布不尽相同,这会导致不同充放电状态下的XRD峰强可比性较差;不同极片在拆卸、洗涤后往往处于不同褶皱状态,所得到的XRD峰则会发生不同程度的偏移。而原位XRD测试的整个过程是对同一个材料的同一片区域位置进行扫描分析,因此得到的信息(无论是晶胞参数、峰强度亦或是其他参数)具有较高的可比性,可以得到一系列实时的结构变化信息,有助于深入认识材料在充放电过程中发生的反应,对如何改进电极材料性能具有实际的指导意义。
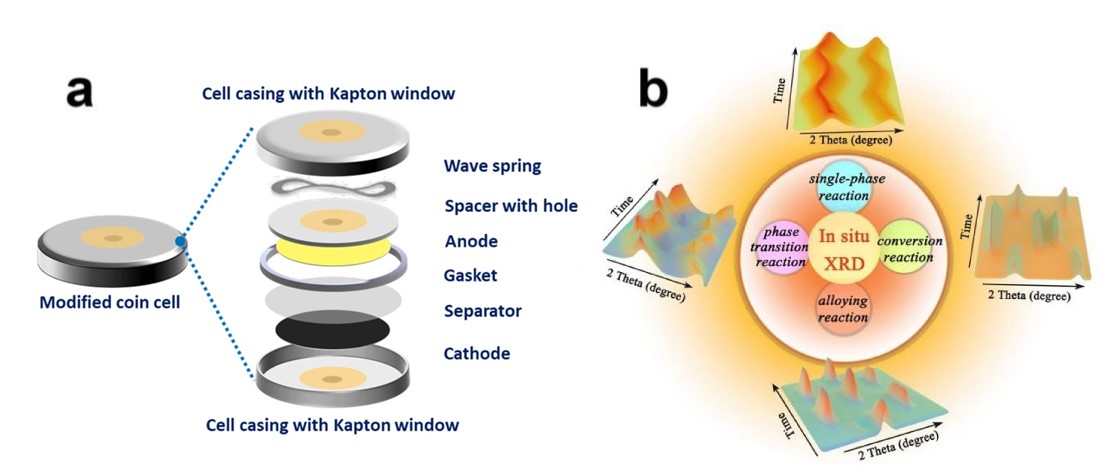

图2原位电池的组装及应用
近日,发表在《ACS Applied Materials & Interfaces》的文章中,吉林大学Chunzhong Wang课题组基于丰富的电池设计及研究经验,用原位XRD表征技术揭示了钠基底上的Ag nanoparticle的electrochemical coating机理:

图3基于多晶XRD数据分析的AgTi2(PO4)3结构精修

图4 (a)第一、二放电过程中原位XRD检测的多晶衍射谱图;在(b)放电及(c)充电过程中,与GCD曲线对应的AgTi2(PO4)3多晶多晶衍射谱。
当电池开始放电时,位于38°的Ag宽峰开始出现,且在放电过程中峰位保持不变。当放电至2.1V时,AgTi2(PO4)3的20.9° (110) and 32.1° (116)逐渐变弱,且伴随着来自于NaTi2(PO4)3的新峰20.3° (110) and 32.4° (116)的出现,此相变过程揭示了相变机制:
AgTi2(PO4)3+ Na++ e-→ NaTi2(PO4)3+ Ag
此外,原位XRD同时完美监测到电池充放电中具有高度可逆性的两相相变过程。
原位XRD能在线监测电池材料的实时结构演化,其高灵敏度与高分辨率在电池材料研究中起到出色的支撑作用。期待原位XRD技术能够在功能材料的研发、结构表征及机理探索中起到关键作用。
Reference
In Situ Electrochemical Coating Mechanism of NASICONStructured AgTi2(PO4)3 for Sodium-Ion Batteries,Zhixuan Wei, Zhongyu Zhang, Nan Chen, Gang Chen, Chunzhong Wang, and Fei Du, ACS Appl. Mater. Interfaces, Page 1-20 Just Accepted Manuscript • DOI: 10.1021/acsami.9b20539 • Publication Date (Web): 09 Jan 2020
Link:https://pubs.acs.org/doi/abs/10.1021/acsami.9b20539
Applications of Lab-Scale in-situ XRD Characterization in Battery Charging/Discharging Study
【Facilities:in-situX-Ray Diffractometor: equipped with rotational anode and 2D Eiger detector】
【Location: XRD lab, Room 112, Building 4】
The XRD lab at ISCPS provides access to state-of-the-art in-situ XRD characterization for batteries, which hosts the diffractometer equipped with large area 2D detector Eiger, temperature-dependent battery chamber as well as powerful data processing software and rich-experience technical support for energy material research at 开云app官网下

Fig 1 the configuration of in-situ XRD and the assembly of battery chamber on it
Due to the ever-increasing desire for portable electronic devices and electrical cars, Li batteries who bears the advantages of high energy density, no memory effect and slow charge loss raise urgent need to optimize their electrochemical performance. The key factor among all in influencing electrochemical performance is the reaction mechanism of electrolytes. Also the electrochemical performance is predominated by their compositions and structures. A series of research focusing on the composition, structure and corresponded property of electrolyte materials, exploring the charging/discharging process of structure evolution, ion and charge transfer are of great significance. In-situ XRD literally concentrates on real-time screening for functional materials on their formation and transformation. For instance, in-situ XRD is capable of real-time monitoring the structure evolution of electrolytes and revealing the reaction mechanism for battery.
Attributed to the influence of physical discrepancy of battery electrode, disassembly procedure, cleaning and transfer procedure of electrode, non in-situ XRD measurement usually failed to reflect the real situation of charging/discharge behavior for batteries. For example, the quality and distribution of effective components on electrode are different, which results in poor reproducibility of peak intensity during various charging/discharging cycles. Moreover, different electrodes maintain different folding mode, which also brings the peak shift in XRD at some extend. In-situ XRD remains scanning the identical area of the target all through the process. Herein, the information of unit cell parameters, peak intensity etc is highly comparable. It sheds light on improving the performance of electrolyte materials by investigating the structure evolution process and electrochemical reactions.


Fig 2 In-situ assembly of batteries and their applications
Recently, the article published onACS Applied Materials & Interfacesfrom Wang group, applied in-situ characterization technique with their novel design and rich experience in battery operation, to reveal an electrochemically Ag nanoparticle coating mechanism upon sodiation, facilitating the electron transfer in the complex.

Fig 3 Rietveld refinement of AgTi2(PO4)3based on the powder XRD

Fig 4 (a) The contour plots of in situ XRD patterns of the selected 2 theta degree ranges during the first and second discharge and charge process; Selected individual diffraction patterns of AgTi2(PO4)3stacked against the GCD potential profile during the first discharge (b) and charge (c) process
Upon discharged to 2.1 V (Figure 5b, Region I), the peaks at 20.9° and 32.1°, corresponding to the (110) and (116) planes of AgTi2(PO4)3, slowly get weakened along with the appearance and enhancement of two new peaks at 20.3° and 32.4°, which can be indexed to the (110) and (116) planes of NaTi2(PO4)3, revealing the replacement reaction between NaTi2(PO4)3 and AgTi2(PO4)3 as follows:
AgTi2(PO4)3+ Na++ e-→ NaTi2(PO4)3+ Ag
Furthermore,in-situXRD study indicates that the corresponding contour plots shown in Figure 4a display two pairs of highly reversible biphasic reactions.
Generally,in-situXRD monitors thereal-timestructuralevolution of battery materials with the capabilities of high sensitivity and elegant resolution. It is significant thatin-situXRD technique plays a key role in structure characterization and reaction machnism investigation for functional material research.
Reference
In Situ Electrochemical Coating Mechanism of NASICONStructured AgTi2(PO4)3 for Sodium-Ion Batteries,Zhixuan Wei, Zhongyu Zhang, Nan Chen, Gang Chen, Chunzhong Wang, and Fei Du, ACS Appl. Mater. Interfaces, Page 1-20 Just Accepted Manuscript • DOI: 10.1021/acsami.9b20539 • Publication Date (Web): 09 Jan 2020
Link: https://pubs.acs.org/doi/abs/10.1021/acsami.9b20539

